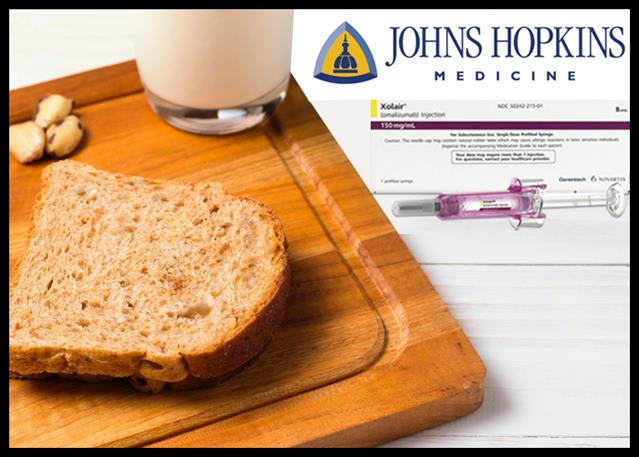
Food allergy is the reaction of the immune system that gets triggered in some people after eating foods containing allergens, say nuts, milk, eggs, soy, wheat, fish, and shellfish. The symptoms can range from digestive problems, hives, swollen airways to something threatening like anaphylaxis that causes the body to go into shock.
Recently, the FDA approved Novartis/Roche’s asthma drug Xolair to reduce the risk of allergic reactions that may occur with accidental exposure to one or more foods in certain adults and children 1 year or older.
A study led by Johns Hopkins Children’s Center has shown that Xolair, known generically as Omalizumab, is effective in reducing life-threatening reactions in people with peanut and other common food allergies.
In this three-stage study that was funded by the National Institutes of Health, investigators compared the effects of 16-20 weeks of Xolair injections with placebo injections in 180 participants, of ages 1 to 55, who had a peanut allergy history along with at least two other food allergies.
After 16 weeks, it was found that 66.9% of the patients who received Xolair were able to tolerate 600 mg or more of peanut protein, which is equivalent to 2.5 peanuts, compared with 6.8% of the participants who received placebo injections.
The study also revealed that Xolair injections raised patients’ reactivity thresholds, not only to peanuts but also to other prevalent food allergies like milk, eggs, wheat, cashews, walnuts, and hazelnuts. These elevated levels provide protection against reactions following inadvertent exposure.
Commenting on the study findings, Robert Wood, director of the Eudowood Division of Allergy, Immunology and Rheumatology at Johns Hopkins Children’s Center, and the study’s principal investigator, said, “This is unique, because we found omalizumab (Xolair) is effective for seven different food allergens. Our findings have the potential to be very meaningful, and potentially even life changing, for people with food allergies.”
The only other FDA-approved treatment for food allergies is Aimmune Therapeutics’ Palforzia, an oral immunotherapy product, indicated for managing peanut allergies in children aged 4 to 17 years.
Copyright © 2024, RTTNews.com, Inc. All Rights Reserved.