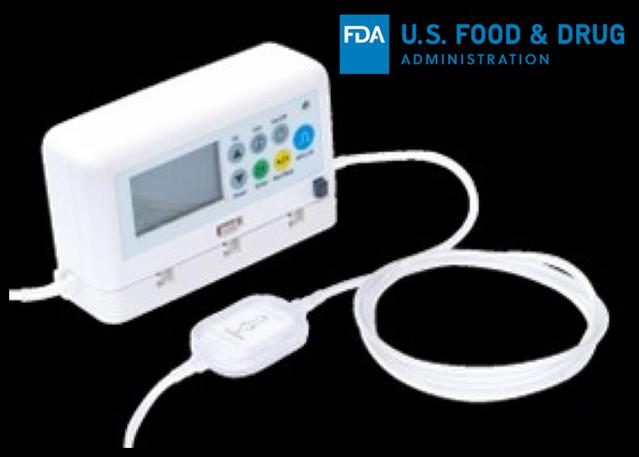
InfuTronix, LLC is recalling Nimbus Ambulatory Infusion Pump System due to several potential product issues following around 3698 customer complaints, according to the U.S. Food and Drug Administration.
The potential product issues were identified following the evaluation of the received complaints related to the Nimbus Infusion Pump systems dated May 2019 to August 2023. The company sees a redesign of the system as the best corrective and preventive action to address the product issues and potential outcomes.
As the redesign may require a new premarket notification and clearance from FDA, InfuTronix has decided to remove the Nimbus Infusion Pump Systems from the market until the changes are made and a clearance is obtained.
Beyond June 20, 2024, the products will not be supported by InfuTronix for either Nimbus Infusion Pumps or related infusion sets.
The recall involves Nimbus II PainPro, Nimbus II Flex, Nimbus II Plus, Nimbus II EpiD and Nimbus II EMS from the US Market.
The affected Nimbus Ambulatory Infusion Pump comes with Unique Device Identification or UDI number of 00817170020000; Nimbus II PainPRO with UDI number 00817170020086; Nimbus II Flex with UDI number 00817170020093; Nimbus II Plus with UDI number 00817170020161; Nimbus II EpiD with UDI number 00817170020376; and Nimbus II EMS with UDI number 00817170020109
The Nimbus Infusion Pump system has been distributed throughout the United States since October 17, 2014 until February 21, 2024. They were not distributed internationally.
Under the several potential product issues, the agency noted that battery power may potentially affect the performance of the pump by causing an immediate power off event. Upstream Occlusion, as noted by the upstream occlusion alarm, occurs when there is a block in flow of the proximal end of the administration set.
Further, there could be, among other issues, System Errors, as noted by the System Error alarm which causes the pump to suspend the infusion.
These product issues were identified through the InfuTronix post-market surveillance system and evaluated through the InfuTronix Corrective Action/Preventive Action system.
Users may continue to use the Nimbus Infusion Pump system and associated infusion sets during the removal process.
In order to return Nimbus products, customers are asked to follow the instructions provided in the Medical Device Removal letter that was sent by InfuTronix, contact InfuTronix customer service or their local distributor.
For More Such Health News, visit rttnews.com
Copyright © 2024, RTTNews.com, Inc. All Rights Reserved.